اکسید پتاسیم
| اکسید پتاسیم | |
|---|---|
| |
Potassium oxide | |
دیگر نامها Potassium monoxide | |
| شناساگرها | |
| شماره ثبت سیایاس | ۱۲۱۳۶-۴۵-۷ |
| شمارهٔ یواِن | 2033 |
| خصوصیات | |
| فرمول مولکولی | K2O |
| جرم مولی | 94.20 g/mol |
| شکل ظاهری | pale yellow solid |
| چگالی | 2.35 g/cm3 |
| دمای ذوب | >۳۵۰ °C decomp. |
| انحلالپذیری در آب | Reacts forming پتاسیم هیدروکسید |
| ساختار | |
| ساختار بلوری | فلئوریت (cubic) cF12 |
| گروه فضایی | Fm3m No. 225 |
| Tetrahedral (K+); cubic (O2–) | |
| خطرات | |
| MSDS | ICSC 0769 |
| شاخص ئییو | Not listed |
| خطرات اصلی | Corrosive, reacts violently with water |
| نقطه اشتعال | |
| ترکیبات مرتبط | |
| دیگر آنیونها | پتاسیم سولفید |
| دیگر کاتیونها | لیتیم اکسید سدیم اکسید روبیدیوم اکسید سزیم اکسید |
| مرتبط با پتاسیم اکسیدs | پتاسیم پراکسید پتاسیوم سوپر اکسید |
| ترکیبات مرتبط | پتاسیم هیدروکسید |
| به استثنای جایی که اشاره شدهاست در غیر این صورت، دادهها برای مواد به وضعیت استانداردشان داده شدهاند (در 25 °C (۷۷ °F)، ۱۰۰ kPa) | |
| | |
| Infobox references | |
|
| |
اکسید پتاسیم یک ترکیب یونی از پتاسیم و اکسیژن است که فرمول شیمیایی K2O را دارا میباشد. پتاسیم را نمیتوان آزاد یافت زیرا بسیار واکنش پذیر است. این ماده دارای ظرفیت +۱ است و به راحتی با اتمهای اکسیژن ترکیب میشود. K2O، هنگام اکسید شدن پتاسیم به عنوان یک ماده کریستالی خاکستری مشاهده میشود. پتاسیم در اکسیژن اضافی سوخته و تشکیل اکسید پتاسیم میدهد.
سادهترین اکسید پتاسیم یک ترکیب بسیار واکنش پذیر است که کمیاب میباشد. برخی از مواد تجاری مانند کودها و سیمانها با فرض درصد ترکیبی که معادل مخلوط ترکیب شیمیایی K2O است در نظر گرفته میشود.
نامهای دیگر این ماده عبارتند از: مونوکسید پتاسیم، هیدروکسید دیپتاسیم و اکسید کالیوم.
تولید[ویرایش]
اکسید پتاسیم از واکنش اکسیژن و پتاسیم تولید میشود. این واکنش حاصل پرواکسید پتاسیم است. عملیات بین پروکسید پتاسیم با پتاسیم منجر به تولید اکسید پتاسیم میشود.[۱]
K2O2 + 2K → 2 K2O
در روشی راحت تر و کاربردی تر اکسید پتاسیم با حرارت دادن نیترات پتاسیم با پتاسیم فلزی سنتز میشود.
2KNO3 + 10 K → 6K2O + N2
روش دیگر گرمایش پروکسید پتاسیم در دمای ۱۵ درجه سانتیگراد است که در آن دما تجزیه شده و اکسید پتاسیم خالص و اکسیژن را میدهد.
2K2O2 → 2K2O + O2
هیدروکسید پتاسیم نمیتواند به اکسید دهیدراته شود. اما میتواند با پتاسیم مذاب واکنش داده و آن را تولید کند و هیدروژن را به عنوان محصول جانبی آزاد کند.
2KOH + 2K → 2K2O + H2

خواص و واکنش[ویرایش]
اکسید پتاسیم یک قلیایی به شدت خوردهاست که در آب حل میشود.
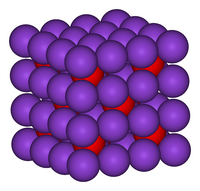
اکسید پتاسیم در ساختار آنتی فلوریت متبلور میشود. در این الگو، موقعیت آنیونها و کاتیونها نسبت به موقعیت آنها در CaF2 معکوس میشود، با یونهای پتاسیم که با ۴ یون اکسید و یونهای اکسید که با ۸ پتاسیم همسایه است.[۲][۳] اکسید پتاسیم یک اکسید بازی است و با آب به شدت واکنش میدهد و هیدروکسید پتاسیم سوزآور تولید میکند. این ماده لطیف کننده است و با شروع این واکنش شدید، آب محیط را جذب میکند.
ویژگی[ویرایش]
یک اکسید سنگین است و بهطور کلی درخشانترین رنگها را به جز سرب میزبانی میکند. برای لعابهای براق و درخشندگی بیشتر و حتی بیشتر از کربنات سدیم ترجیح داده میشود. پلاکهای رنگی بسیار خوبی را میتوان در فرمولهای غالب K2O - PbO - SiO2 ساخت.
اکسید پتاسیم یک شار کمکی مهم در لعابهای با درجه حرارت بالا است.
به عنوان یک اکسید بسیار پایدار و قابل پیشبینی در نظر گرفته شدهاست.
آسیبها[ویرایش]
استنشاق، بلع یا تماس پوست با مواد ممکن است باعث آسیب شدید یا مرگ شود. تماس با ماده مذاب ممکن است باعث سوختگی شدید پوست و چشم شود. از هرگونه تماس پوستی خودداری کنید. آثار تماس یا استنشاق ممکن است با تأخیر همراه باشند. آتش ممکن است گازهای تحریک کننده، خورنده و / یا سمی تولید کند. رواناب حاصل از کنترل آتش یا رقت آب ممکن است خورنده یا سمی باشد و باعث آلودگی شود.
خطر سوختن[ویرایش]
ماده غیرقابل احتراق به خودی خود نمیسوزد اما ممکن است هنگام گرم شدن تجزیه شود و بخارات خورنده و / یا سمی تولید کند. برخی از آنها اکسید کننده هستند و ممکن است مواد قابل احتراق (چوب، کاغذ، روغن، لباس و غیره) را مشتعل کنند. تماس با فلزات ممکن است باعث ایجاد گاز هیدروژن قابل اشتعال شود. ظروف ممکن است هنگام گرم شدن منفجر شوند
کمکهای اولیه[ویرایش]
اطمینان حاصل کنید که پرسنل پزشکی از ماده مربوط آگاهی دارند و برای محافظت از خود اقدامات احتیاطی را انجام میدهند. حرکت مصدوم به هوای تازه انجام شود. با فوریتهای پزشکی تماس بگیرید. دادن تنفس مصنوعی اگر قربانی نفس نمیکشد. اگر قربانی ماده را بلعید یا استنشاق میکند از روش دهان به دهان استفاده نکنید. تنفس مصنوعی را با کمک ماسک جیبی مجهز به دریچه یک طرفه یا سایر تجهیزات پزشکی تنفسی مناسب انجام دهید. در صورت دشوار بودن تنفس اکسیژن مصرف کنید. کفشها و لباسهای آلوده را درآورید و قرنطینه کنید. در صورت تماس با ماده، پوست یا چشمها را بلافاصله حداقل ۲۰ دقیقه با آب بشویید. برای تماس پوستی جزئی، از پخش مواد روی قسمت پوست تمیز خودداری کنید. مصدوم را آرام و گرم نگه دارید.
واکنش هوایی و آبی[ویرایش]
محلول در آب. اکسیدهای پتاسیم با آب به شدت و افزایش حرارت و گرما واکنش نشان میدهند تا جایی که جوش و پاشیدن محلول گرم سوزان شود.
بخش استفاده در صنعت[ویرایش]
فرمول شیمیایی K2O (یا به سادگی "K") در چندین زمینه صنعتی استفاده میشود: N-P-K برای کودها، فرمولهای سیمان و فرمولهای شیشه سازی.
از اکسید پتاسیم اغلب بهطور مستقیم در این محصولات استفاده نمیشود، اما مقدار پتاسیم بر حسب معادل K2O برای هر نوع پتاس مانند کربنات پتاسیم استفاده میشود. به عنوان مثال، اکسید پتاسیم از نظر وزنی حدود ۸۳٪ پتاسیم است در حالی که کلرید پتاسیم تنها دارای ۵۲٪ است. کلرید پتاسیم، پتاسیم کمتری نسبت به مقدار مساوی در اکسید پتاسیم تأمین میکند؛ بنابراین، اگر کودی از نظر وزنی دارای ۳۰٪ کلرید پتاسیم باشد، میزان استاندارد پتاسیم آن بر اساس اکسید پتاسیم، فقط ۱۸٫۸٪ خواهد بود.
اکسید پتاسیم همراه سدیم نیز زیاد استفاده میشود، زیرا این دو تقریباً همیشه با هم اتفاق میافتند و خواص بسیار مشابه ای دارند. هنگامی که این دو با هم جمع میشوند اغلب برچسب KNaO دارند. اکسید پتاسیم بهطور کلی ویسکوزیته ذوب بالاتری نسبت به Na2O ایجاد میکند.
در صنایع مختلف دیگر کاربردهای گستردهای دارد. از جمله:
جاذبها
کاتالیزور
سنگریزه و مواد بستر جاده
واسطهها
تنظیم کنندههای فرایند
کمکهای فرایند و …
در شیشه سازی[ویرایش]
به غیر از اینکه در شیشههای صنعتی کاربرد دارد، در تولید شیشه سرامیکهای دندانی لئوسایتی و در ساختار لئوسایت به عنوان ماده اصلی شناخنه میشود. که البته به خاطر کمیاب بودن اکسید پتاسیم خالص، در فرایندها از نیتراتها، کربناتها و دیگر مشتقات آن استفاده میشود.
در ابتدا در دمای بالای ۱۰۰۰ درجه سانتیگراد ذوب بچ انجام میگیرد، سپس با تعیین دمای کریستالیزاسیون برای متبلور شدن لئوسایت، عملیات حرارتی انجام میگردد که میزان اکسید پتاسیم در این مرحله مهم است.
در پزشکی[ویرایش]
برای درمان گرانولوماتوز قارچی و عفونتهای مرتبط با بیماری zygomycetes استفاده میشود.
بیش از ۱۰۰ سال در درمان اکتینومایکوزیس و اکتینوباسیلوز در گاو استفاده میشود.
همچنین در درمان اسم و بوتریومیکوز استفاده میشود.
در مصارف کود[ویرایش]
از آنجا که پتاسیم یکی از مهمترین عناصر مورد نیاز گیاهان است، عمدهترین مصرف پتاس در تهیه کود شیمیایی است. حدود ۹۵٪ از تولید حهانی پتاس در صنایع کشاورزی به منظور تهیه کود مورد نیاز گیاه به کار میرود. این کود پتاسیم نوعی کود شیمیایی با ۵۰ تا ۵۲ درصد K یا (۶۰ تا ۶۸ درصد) K2O است و رنگ آن از صورتی یا قرمز تا قهوهای یا سفید متغیر بوده، بهطوری که رنگ آن وابسته به فرایند استخراج و بازیافت میباشد.
منابع[ویرایش]
- ↑ Holleman, A. F. ; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. شابک ۰−۱۲−۳۵۲۶۵۱−۵.
- ↑ Brauer, G.; Zintl, E. (1937-01-01). "Konstitution von Phosphiden, Arseniden, Antimoniden und Wismutiden des Lithiums, Natriums und Kaliums". Zeitschrift für Physikalische Chemie. 37B (1). doi:10.1515/zpch-1937-0125. ISSN 2196-7156.
- ↑ Wells, A.F. (1984) Structural Inorganic Chemistry, Oxford: Clarendon Press. ISBN 0-19-855370-6.
